Nitrosamines - What are they? Why are they a problem for drug manufacturers? And what is now required of EU Marketing Authorisation Holders?
As nitrosamines continue to feature heavily in the context of EU drug product CMC considerations, G&L’s Salli Awad, Michael Sweeney and Sandra Maguire look at the story so far and the implications for changes due imminently…
The deadline for completing Step 2 confirmatory testing for chemical medicines under Article 5(3) is 26 September 2022.
Fortunately for Marketing Authorization Holders, the EU Committee for Medicinal Products for Human Use (CHMP) and Co-ordination Group for Mutual Recognition and Decentralised procedures – Human (CMDh) have extended the deadline for submitting Article 5(3) variation applications for chemical medicines from September 2022 to October 2023.
The extension aims to enable companies to perform a thorough investigation and to establish any required risk mitigating actions in light of new scientific developments since 2020.
A global review into the level of nitrosamines in medicinal products started in 2018, with the initial revelation that nitrosamines were found in the sartans family of drugs used to treat heart disease1.
Stemming from this, an industry-wide review of all medicines (including factors such as drug substance, excipients and packaging materials) was implemented to evaluate for the possible presence of nitrosamines.
What are nitrosamines?
Nitrosamines (Figure 1.) are a family of organic compounds that have been identified as carcinogenic (i.e., having the potential to cause cancer).
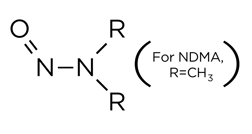
Figure 1. Chemical Structure of Nitrosamine
The most prevalent is nitrosodimethylamine (NDMA), although many different nitrosamines can be formed depending on the R group of the parent amine (R=Et, iPr, etc).
Secondary (2°) amines are the most susceptible to undergoing a reaction to form a nitrosamine (nitrosation reaction), although any primary amine has to be considered a potential risk as secondary amines may exist as impurities.
Tertiary amines may degrade into 2° amines and undergo nitrosation. Dimethyl-formaldehyde (DMF), which is a very common organic solvent used in many synthetic processes, may degrade to form a 2°amine which will then form NDMA (Figure 2.).2,3
The other reagent needed for nitrosation to occur is a nitrite (NOx). The most common is sodium nitrite (NaNO2), but any NOx is a possible precursor to a nitrosation reaction.
Nitrosamines can also be found in food and drinking water4 in minimal quantities. Health authorities around the world have different limits, but all range from (0.6 ng/L to 10ng/L).5
Levels are continually monitored to ensure that nitrosamines’ levels do not rise above an acceptable intake (AI) and pose a greater than the lifetime risk of cancer (1 in 100,000)6.
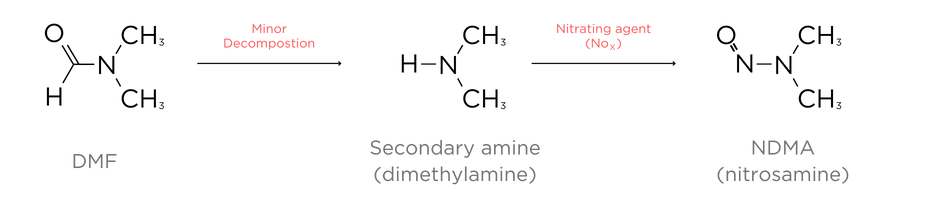
Figure 2. Simplified pathway for decomposition of DMF to form NDMA
The impacts on EU Marketing Authorization Holders
The CHMP Article 5(3) concluded its referral on nitrosamines in September 2019.
It established that nitrosamines should be classified as carcinogens and that their potential presence in medicines posed a risk to patients. Therefore, marketing authorisation holders (MAHs) should review their medicines to identify nitrosamine impurities and, if found, mitigate their risk.5,6
The European Medicines Regulatory Network (EMRN) subsequently published the approach for the implementation of the CHMP Opinion under Article 5(3) of Regulation (EC) No 726/2004 for nitrosamine impurities in human medicines (EMA/425645/2020).
This process comprised the following three stages:
Step 1: Risk evaluation
MAHs were required to perform and submit a risk evaluation for each medicine for which they have a marketing authorisation.
The purpose of this was to identify if the active substances and/or finished products are at risk of nitrosamine(s) impurity formation and report the outcome to EMA/EU national authorities6.
The risk assessment included an evaluation of all potential sources of nitrosamines, such as the drug substance synthesis, excipients, finished product manufacturing processes, cross-contamination, cleaning procedures, packaging, and printing inks7.
The outcome of this stage was either a risk of nitrosamines being present or a declaration of absence of risk of nitrosamine contamination.
Step 2: Confirmatory testing
For medicines where a risk of nitrosamine(s) formation was identified, confirmatory testing is required7.
For this purpose, it is necessary to develop and validate appropriate analytical testing methods, and the testing of a representative number of drug substances and/or finished product batches to confirm the presence of nitrosamine(s) and, if present, the level of contamination found7.
In case of a confirmed risk, the nitrosamine level measured should be compared with a calculated acceptable intake (AI) limit and a risk level of 1:100,000 applied. In cases where the quantity of nitrosamine(s) measured is below 10% of the AI limit or the risk of 1:100,000, no further action is required.
However, where the nitrosamine(s) level measured is greater than 10% of any of these limits, routine testing for nitrosamine(s) and/or mitigation of risk, such as changing the processes and/ or sources causing nitrosamine(s) formation, must be implemented by the MAH. 6,7
A newly identified nitrosamine that is not covered in CHMP article 5 (3) opinion should be declared, irrespective of the amount detected.6
Further and specific guidance is available for medicines where there is a known risk of nitrosamine(s) formation and includes sartan, rifampicin, ranitidine, metformin, varenicline containing medicines as described in the European Medicines Agency document EMA/409815/2020.6,7
Step 3: Update marketing authorisations (deadline update)
Marketing authorisation holders must apply for any necessary changes which have resulted from the nitrosamine(s) impurities review by requesting a variation to the marketing authorisations via standard regulatory processes6.
Deadline for completion of steps
The deadlines for each step are outlined in the table below:
| Step Number | Chemical Medicinal Products | Biological Medicinal Products |
| Step 1 – Risk evaluation | 31 March 2021 | 01 July 2021 |
| Step 2 – Confirmatory testing | 26 September 2022 | 01 July 2023 |
| Step 3 – Submission of relevant changes |
01 October 20236 | 01 July 20236 |
Current ramifications of the review
Globally, several medicines have been withdrawn from the market due to the presence of nitrosamines, notably ranitidine,8 an antacid drug (one trade name, Zantac), which was found to build high levels of nitrosamines.
In 2021, ‘Zantac 360’9 was released. This product replaces ranitidine with famotidine as the active ingredient, a compound with similar pharmacological properties but no evidence of nitrosamine risk, which has been approved by The Food and Drug Administration (FDA) in the USA.10
In Europe, the EMA has obligated the routine testing of nitrosamines, in metformin (NDMA) and rifamipicin (1-nitroso-4-methyl piperazine) containing medicines, while investigations are ongoing 7.
For other medicines, the marketing authorisation holder recalled several batches and paused the distribution of Champix (varenicline), a smoking cessation medicine, since June 2021 because of the presence of N Nitroso-varenicline at a level above the AI set by EMA - this has led to an EU-wide shortage of this medicine4
As the deadline for nitrosamines confirmatory testing of chemical medicinal products approaches, it remains to be seen if any other drug products will require reformulation, or worse, withdrawal from the market.
References
1. J. Leclerc, Canadian Journal of Cardiology, 2018, 34, 1370.e13.
2. J. Muzart, Tetrahedron, 2009, 65, 8313–8323.
3. P. Andrzejewski, B. Kasprzyk-Hordern and J. Nawrocki, Water Res., 2008, 42, 863–870.
4. C.-C. Fan and T.-F. Lin, Chemosphere, 2018, 200, 48–56.
5. CHMP Assessment Report for Nitrosamine impurities in human medicinal products - Procedure under Article 5(3)of Regulation EC (No) 726/2004 (EMA/369136/2020).
6. EMA, Nitrosamine impurities | European Medicines Agency, https://www.ema.europa.eu/en/human-regulatory/post-authorisation/referral-procedures/nitrosamine-impurities#guidance-for-marketing-authorisation-holders-section, (accessed June 21, 2022).
7. European Medicines Agency, EMA/409815/2020 Rev.10.
8. FDA, FDA Requests Removal of All Ranitidine Products (Zantac) from the Market | FDA, https://www.fda.gov/news-events/press-announcements/fda-requests-removal-all-ranitidine-products-zantac-market, (accessed June 21, 2022).
9. Popular heartburn medication returns to market with new name - Zantac 360°TM - and new active ingredient, https://workcompauto.optum.com/content/owca/owca/en/insights/blog/clinical-connection-blog/2021/Heartburn-medication-returns-to-market-with-new-name-Zantac-360.html, (accessed July 11, 2022).
10. FDA, FDA Approved Drug Product List, https://www.fda.gov/media/71494/download, (accessed June 21, 2022).